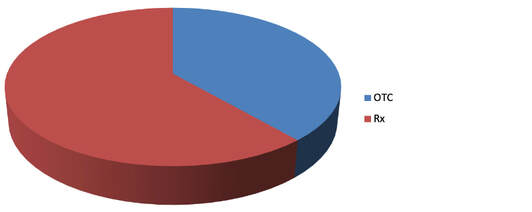
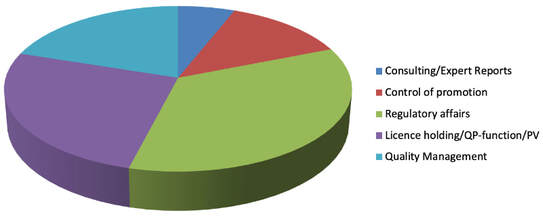
Portfolio in numbers
In the last > 20 years we have gained marketing authorisation approvals for medicinal products of the following therapeutic classes.
|
|
|
Licensed Products
Currently we are licence holder (MAH) of the following medicinal products, on behalf of our clients:
Sintrom 1mg tablets |
Pevaryl(R), creme |
Sintrom 4mg tablets |
Pevaryl(R), powder |
Esidrex(R) 25 mg, tablets |
Pevaryl(R), pump spray |
Insidon(R), coated tablets |
Dacepton(R) 10 mg/ml, inj. sol. |
Entumin(R) 40 mg, tablets |
Dacepton(R) 5 mg/ml, inj. sol. |
Arimidex, Film-coated tablets |
Oramorph 10 to 100 mg/5 mL, sol. |
Casodex, film-coated tablets |
Oramorph 20 mg/1 mL, sol. |
Pylera, capsules |
Latuda(R) 20, 40 , 80, 120 mg, tablets |
Qarziba, concentrate for solution for injection, inj. suspension |
Sylvant, 100mg and 400mg powder for inf. |
Foclivia, inj. susp. |
Scheriproct, ointment |
Pevisone, crème |
Gyno-Pevaryl range |
Scheriproct, Suppositories |
Betoptic-S range, suspension |
Lymphoseek, kit |
Emadine / Emadine SE, eye drops |
Venostasin, ointment |
Venostasin, capsules |
Aulin 100, granules |
Phlebostasin, capsules |
Aulin 100, tablets |
Akynzeo 300mg/0.5mg capsules |
Aloxi, inj. |
Akynzeo IV 235mg/0.25mg powder for inj. |
Aloxi 500, capsules |
Feraccru, capsules |
Letybo, powder for inj. |
Pliaglis, creme |
Ben-u-ron, suppositories |
Ben-u-ron, syrup |
Thrombocid, Gel |
Fulphila (pegfilgrastim) solution for injection in pre-filled syringe |
Hulio (adalimumab) solution for injection in pre-filled pen |
Hulio (adalimumab) solution for injection in a vial |
Hulio (adalimumab) solution for injection in pre-filled syringe |
Nepexto (etanerceptum) solution for injection in pre-filled syringe 50 mg and 25mg |
Nepexto (etanerceptum) solution for injection in pre-filled pen 50mg |
Ogivri (trastuzumabum) powder for concentrate for solution for infusion 150mg |
Ogivri (trastuzumabum) powder for concentrate for solution for infusion 440mg |
Ubistesin range (mite, forte) solution for injection |
Lamisil cream |
Lamisil Pedisan cream |
Lamisil Pedisan Once |
Lamisil Pedisan Spray |
Value |
Please refer to Swissmedic homepage for further information regarding the mentiones medicinal products under www.swissmedicinfo.ch
More than 25 years on the market - over 100 years of experience combined.