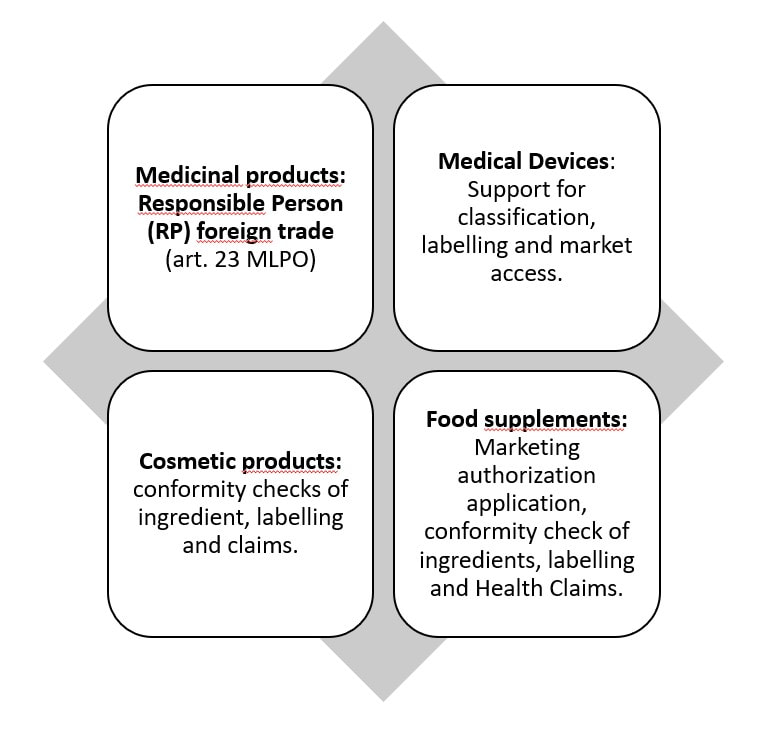
Our solution for market entry
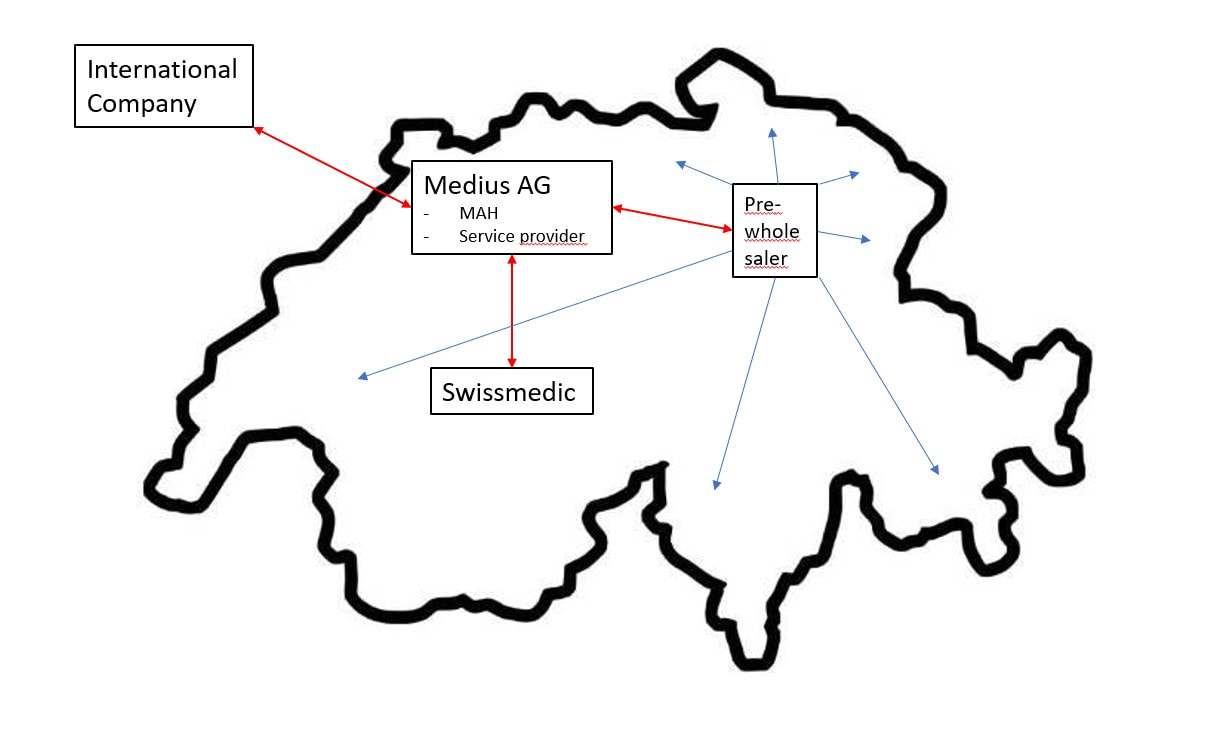
If you are an international company, with no subsidiary in Switzerland, your partner Medius can act on your behalf.
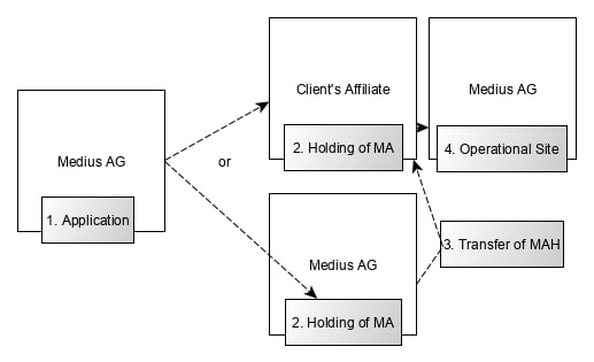
Various options are possible:
|
Contact us to develop the best strategy for your success.
|
Medius is the holder of an establishment license. As your partner we can apply for the Marketing Authorization on your behalf.
|
Medius can act as the licence holder on long-term or as interim solution.
|
Medius can act as the licence holder and transfer the licence any time as required
by your company. |